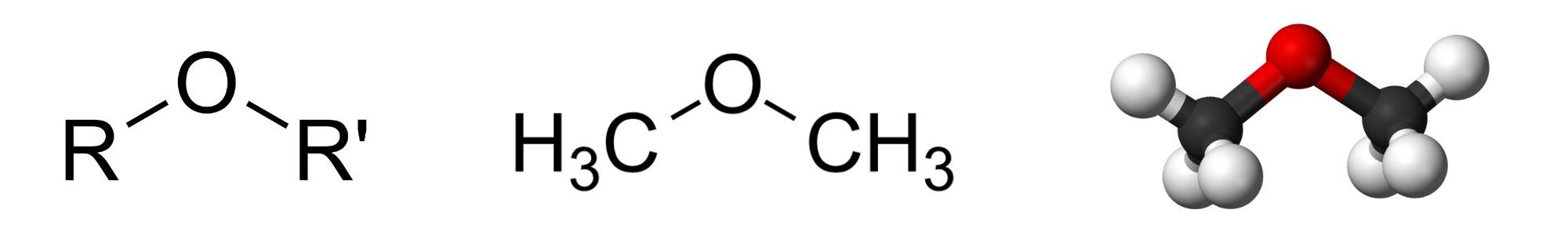
With the general formula ROR ′, an ether may be considered a derivative of water in which both hydrogen atoms are replaced by alkyl or aryl groups (aryl means aromatic). It may also be considered a derivative of an alcohol ( ROH ) in which the hydrogen atom of the OH group is replaced by a second alkyl or aryl group (Figure 23.6a.).

The IUPAC naming process of naming ethers involves separately naming each of the two groups attached to the oxygen atom.
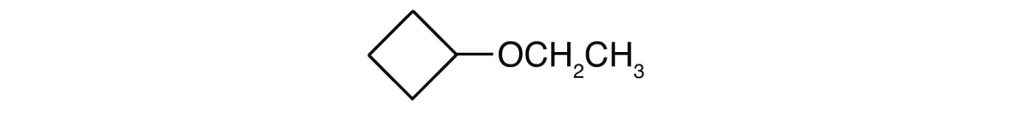
What is the IUPAC name for each ether?
What is the IUPAC name for each ether?
Simple ethers also have common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether. If both groups are the same, the group name should be preceded by the prefix di-.
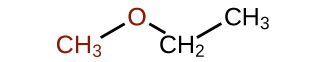
What is the common name for each ether?
What is the common name of this ether?
Source: Exercise 23.6b is adapted from General Chemistry 1 & 2 , CC BY 4.0 .
Ether molecules have no hydrogen atom on the oxygen atom (that is, no OH group). Therefore, there is no intermolecular hydrogen bonding between ether molecules. As a result, ethers have quite low boiling points for a given molar mass. In fact, ethers have boiling points about the same as those of alkanes of comparable molar mass and much lower than those of the corresponding alcohols (Table 23.6a.).
| Condensed Structural Formula | IUPAC Name | Common Name | Molar Mass | Boiling Point (°C) | Intermolecular Hydrogen Bonding in Pure Liquid? |
|---|---|---|---|---|---|
| CH3CH2CH3 | propane | propane | 44 | –42 | no |
| CH3OCH3 | methoxymethane | dimethyl ether | 46 | –25 | no |
| CH3CH2OH | ethanol | ethyl alcohol | 46 | 78 | yes |
| CH3CH2CH2CH2CH3 | pentane | pentane | 72 | 36 | no |
| CH3CH2OCH2CH3 | ethoxyethane | diethyl ether | 74 | 35 | no |
| CH3CH2CH2CH2OH | butan-1-ol | butyl alcohol | 74 | 117 | yes |
Ether molecules do have an oxygen atom, however, and engage in hydrogen bonding with water molecules. Consequently, an ether has about the same solubility in water as the alcohol that is isomeric with it. For example, dimethyl ether and ethanol (both having the molecular formula C2H6O) are completely soluble in water, whereas diethyl ether and 1-butanol (both C4H10O) are barely soluble in water (8 g/100 mL of water).
A general anesthetic acts on the brain to produce unconsciousness and a general insensitivity to feeling or pain. Diethyl ether or ethoxyethane (CH3CH2OCH2CH3) was the first general anesthetic to be used. Diethyl ether is a colourless, volatile liquid that is highly flammable.
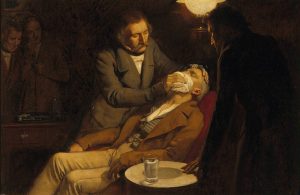
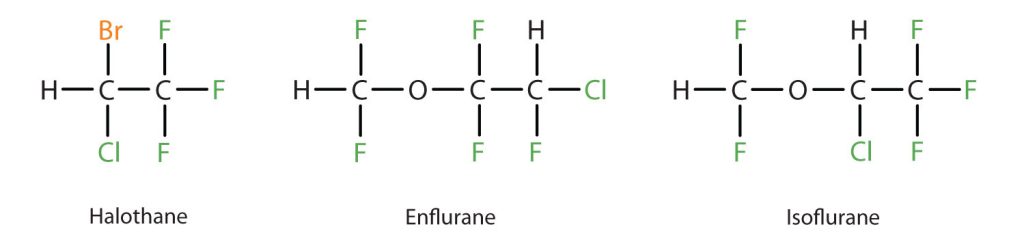
Diethyl ether is relatively safe because there is a fairly wide gap between the dose that produces an effective level of anesthesia and the lethal dose. However, because it is highly flammable and has the added disadvantage of causing nausea, it has been replaced by newer inhalant anesthetics, including the fluorine-containing compounds halothane, enflurane, and isoflurane (Figure 23.6c.). Unfortunately, the safety of these compounds for operating room personnel has been questioned. For example, female operating room workers exposed to halothane suffer a higher rate of miscarriages than women in the general population.
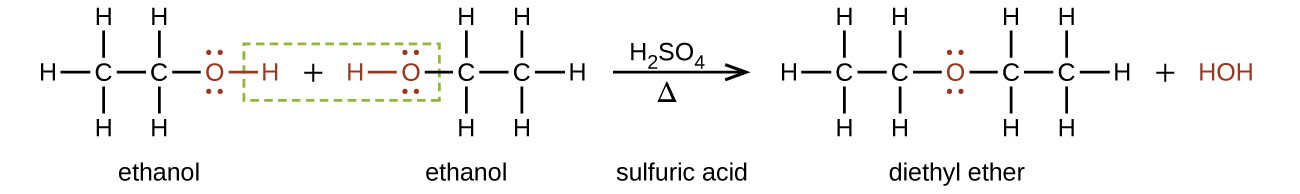
Ethers are produced from alcohols as previously described in this chapter. Figure 23.6d. shows an example of ether production.
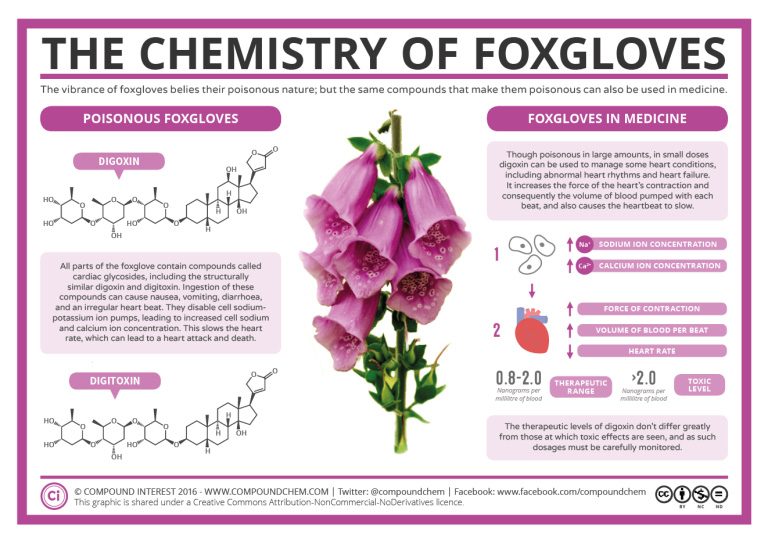
Foxgloves are poisonous. The large biological molecules contained in the flowers have many ether functional groups. Read more about the chemistry of foxgloves in Infographic 23.6a.
For more support with naming ethers and their properties, see Ether naming and introduction (video) | Khan Academy.
Except where otherwise noted, this page is written and adapted by David Wegman and Samantha Sullivan Sauer from
organic compound with an oxygen atom that is bonded to two carbon atoms
× Close definitionOrganic and Biochemistry Supplement to Enhanced Introductory College Chemistry Copyright © 2024 by Gregory Anderson; Caryn Fahey; Adrienne Richards; Samantha Sullivan Sauer; David Wegman; and Jen Booth is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted.